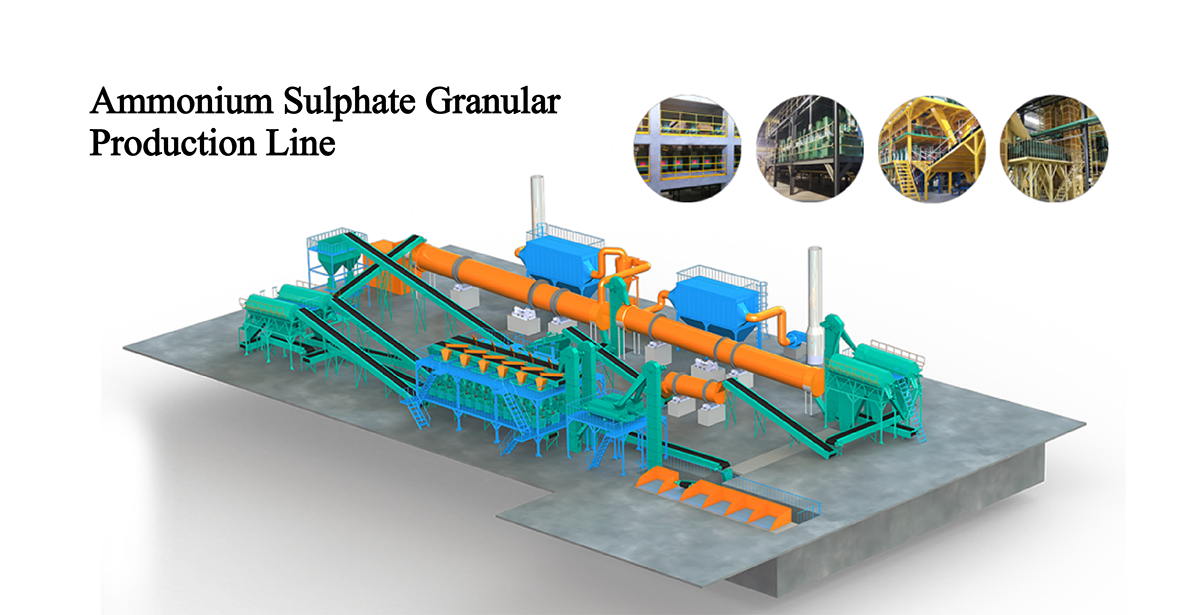
Ammonium Sulphate Capro Crystal
Ammonium sulfate
Name:Ammonium Sulphate(IUPAC-recommended spelling; also ammonium sulphate in British English), (NH4)2SO4, is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer. It contains 21% nitrogen and 24% sulfur.
Other Name: Ammonium Sulfate, Sulfato de Amonio, AmSul, Diammonium Sulfate, Sulfuric Acid Diammonium Salt, Mascagnite, Actamaster, Dolamin.

Nitrogen: 21% Min.
Sulphur: 24% Min.
Moisture: 0.2% Max.
Free Acid: 0.03% Max.
Fe: 0.007% Max.
As: 0.00005% Max.
Heavy Metal(As Pb): 0.005% Max.
Insoluble: 0.01 Max.
Appearance: White or Off-White Crystal
Standard: GB535-1995
1. Ammonium Sulphate is mostly used as nitrogen fertilizer. It provides N for NPK. It provides an equal balance of nitrogen and sulphur, meets short term sulphur deficits of crops, pastures and other plants.
2. Fast release, quick acting;
3. More efficiency than urea, ammonium bicarbonate, ammonium chloride,ammonium nitrate;
4. Can be readily blended with other fertilisers. It has the desirable agronomic features of being a source of both nitrogen and sulphur.
5. Ammonium sulphate can make crops thrive and improve fruit quality and yield and strengthen resistance to disaster,can be used for common soil and plant in basic fertilizer, additional fertilizer and seed manure. Suitable for the rice seedling, paddy fields, wheat and grain, corns or maize, the growth of tea, vegetables, fruit trees, hay grass, lawns, turf and other plants.









The primary use of ammonium sulfate is as a fertilizer for alkaline soils. In the soil the ammonium ion is released and forms a small amount of acid, lowering the pH balance of the soil, while contributing essential nitrogen for plant growth. The main disadvantage to the use of ammonium sulfate is its low nitrogen content relative to ammonium nitrate, which elevates transportation costs.
It is also used as an agricultural spray adjuvant for water-soluble insecticides, herbicides, and fungicides. There, it functions to bind iron and calcium cations that are present in both well water and plant cells. It is particularly effective as an adjuvant for 2,4-D (amine), glyphosate, and glufosinate herbicides.
-Laboratory Use
Ammonium sulfate precipitation is a common method for protein purification by precipitation. As the ionic strength of a solution increases, the solubility of proteins in that solution decreases. Ammonium sulfate is extremely soluble in water due to its ionic nature, therefore it can "salt out" proteins by precipitation. Due to the high dielectric constant of water, the dissociated salt ions being cationic ammonium and anionic sulfate are readily solvated within hydration shells of water molecules. The significance of this substance in the purification of compounds stems from its ability to become more so hydrated compared to relatively more nonpolar molecules and so the desirable nonpolar molecules coalesce and precipitate out of the solution in a concentrated form. This method is called salting out and necessitates the use of high salt concentrations that can reliably dissolve in the aqueous mixture. The percentage of the salt used is in comparison to the maximal concentration of the salt in the mixture can dissolve. As such, although high concentrations are needed for the method to work adding an abundance of the salt, over 100%, can also oversaturate the solution, therefore, contaminating the nonpolar precipitate with salt precipitate. A high salt concentration, which can be achieved by adding or increasing the concentration of ammonium sulfate in a solution, enables protein separation based on a decrease in protein solubility; this separation may be achieved by centrifugation. Precipitation by ammonium sulfate is a result of a reduction in solubility rather than protein denaturation, thus the precipitated protein can be solubilized through the use of standard buffers.[5] Ammonium sulfate precipitation provides a convenient and simple means to fractionate complex protein mixtures.
In the analysis of rubber lattices, volatile fatty acids are analyzed by precipitating rubber with a 35% ammonium sulfate solution, which leaves a clear liquid from which volatile fatty acids are regenerated with sulfuric acid and then distilled with steam. Selective precipitation with ammonium sulfate, opposite to the usual precipitation technique which uses acetic acid, does not interfere with the determination of volatile fatty acids.